
For problematic ligations or if the DNA concentration is unknown, it may be necessary to vary ratios and run multiple ligations. A ratio above 6:1 will promote the insertion of multiple fragments, while dropping below 2:1 will reduce ligation efficiency. Single-insert ligations are optimal when targeting an insert:vector ratio between 2 and 6. It is recommended that the reaction buffer be discarded after one year of storage at -20☌ and replaced with fresh buffer to ensure maximum performance. One Weiss Unit is approximately equivalent to 22 Enzymatics cohesive end units. One Enzymatics T4 DNA Ligase cohesive end unit is equivalent to approximately 3 cohesive end units as measured with a Lambda-Hind III DNA fragment substrate in 1X T4 DNA Ligase reaction buffer. Based on the correlation between the no template control C t values and standard curve data, the detection limit of this assay is <10 copies genome/sample. The acceptance criterion for the test is the threshold cycle count (C t) produced by the average of 3 replicate no template control samples. Replicate 5 µL samples of enzyme solution were denatured and screened in a TaqMan qPCR assay for the presence of contaminating E.coli genomic DNA using oligonucleotide primers corresponding to the 16S rRNA locus. The acceptance criteria for this test requires that the aggregate mass of contaminant bands in the concentrated sample do not exceed the mass of the protein of interest band in the dilute sample, confirming greater than 99% purity of the concentrated sample.Ī 50 µL reaction containing 10,000 cpm of a radiolabeled single-stranded DNA substrate and 10 µL of enzyme solution incubated for 4 hours at 37☌ resulted in less than 1.0% release of TCA-soluble counts.Ī 50 µl reaction containing 5,000 cpm of a radiolabeled double-stranded DNA substrate and 10 µL of enzyme solution incubated for 4 hours at 37&C resulted in less than 1.0% release of TCA-soluble counts.Ī 50 µL reaction containing 0.5 µg of pENZuC DNA and 10 µL of enzyme solution incubated for 4 hours at 37☌ resulted in no visually discernible conversion to nicked circular DNA as determined by agarose gel electrophoresis. Following electrophoresis, the gel was stained and the samples compared to determine physical purity. SDS-Page (Physical Purity Assessment) and SpecificationsĢ.0 µL of concentrated enzyme solution was loaded on a denaturing 4-20% Tris-Glycine SDS-PAGE gel flanked by a broad-range MW marker and 2.0 µL of a 1:100 dilution of the same enzyme species. The observed average measurement of 3 replicate samples was converted to mg/mL using an extinction coefficient of 54,050 and molecular weight of 55,292 Daltons. Protein Concentration (OD 280) MeasurementĪ 2.0 µL sample of enzyme was analyzed at OD 280 using a Nanodrop ND-1000 spectrophotometer standardized using a 2.0 mg/ml BSA sample (Pierce Cat #23209) and blanked with product storage solution. Reactions are incubated for 30 minutes at 23☌, stopped, and analyzed on a 1% agarose gel stained with ethidium bromide. Dilutions of enzyme were made in 1X T4 DNA Ligase Reaction Buffer and added to 20 µL reactions containing double stranded DNA fragments and 1X T4 DNA Ligase Reaction Buffer.
#Klenow fragment ligation serial#
Unit activity was measured using a 2-fold serial dilution method. * For a detailed summary of assay conditions and data, refer to the Quality Controls Analysis section.

PRODUCT SPECIFICATION* Storage Temperature coli strain carrying the cloned T4 DNA Ligase gene.ġ unit is defined as the amount of T4 DNA Ligase required to join 50% of 100 ng of DNA fragments with cohesive termini in 50 µl 1X T4 DNA Ligase Buffer following a 30 minute incubation at 23☌. The enzyme efficiently joins blunt and cohesive ends and repairs single stranded nicks in duplex DNA, RNA or DNA/RNA hybrids (1).Ī recombinant E.
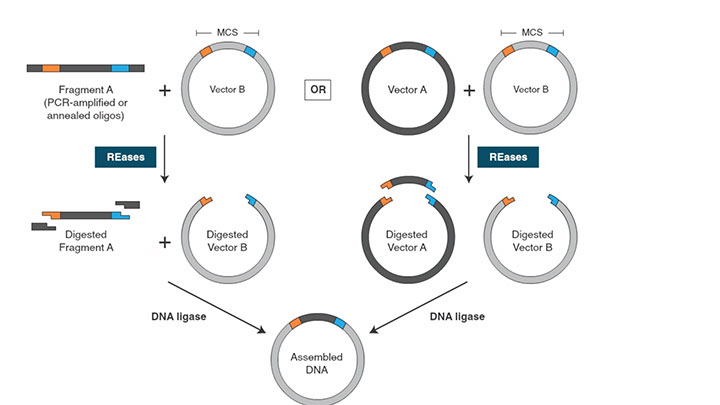
T4 DNA Ligase catalyzes the formation of a phosphodiester bond between the terminal 5′ phosphate and a 3′ hydroxyl groups of duplex DNA or RNA.


 0 kommentar(er)
0 kommentar(er)
